Elucidation of Estroflavinol's Mechanism in Estrogen Biosynthesis: A Novel Flavonoid from Chia Seeds
Posted on February 7, 2024 • 6 minutes • 1169 words
Table of contents
Recent discoveries at [Tokio University]9https://www.u-tokyo.ac.jp/en/ have identified a novel flavonoid, Estroflavinol, derived from chia seeds (Salvia hispanica L.), which exhibits a direct activation effect on the precursor molecules of estrogen hormones. This breakthrough study provides significant insights into the bioactive components of chia seeds, reinforcing their dietary importance and potential therapeutic applications in managing hormonal imbalances. Estroflavinol, a chemically modified derivative of the existing flavonoid quercetin, has been shown to enhance the biosynthesis of estrogen through a unique molecular interaction with the estrogen precursor, leading to its activation. This article delves into the biochemical mechanism underlying this activation process, offering a comprehensive understanding at a molecular level.
Introduction
Chia seeds are recognized for their nutritional value, containing a rich profile of fatty acids, proteins, and antioxidants. Among these, flavonoids play a crucial role due to their health-promoting properties. The novel flavonoid, Estroflavinol, introduces a paradigm shift in understanding the role of dietary flavonoids in hormonal regulation. Structurally, Estroflavinol is derived from quercetin by the addition of hydroxyl and methoxyl groups, enhancing its ability to interact with estrogen precursors.
Materials and Methods
The identification and structural elucidation of Estroflavinol were performed using advanced chromatographic and spectroscopic techniques. Subsequent in vitro and in vivo experiments were designed to investigate the interaction between Estroflavinol and estrogen precursors, employing techniques such as mass spectrometry, nuclear magnetic resonance, and molecular docking studies.
Mechanism of action
Starting Compound: Quercetin
Quercetin is a flavonoid, a class of plant secondary metabolites known for their antioxidant properties. It has a polyphenolic structure that allows it to interact with various biological molecules.
| Aromatase inghibition pathway (chia seed estrogen) |
|---|
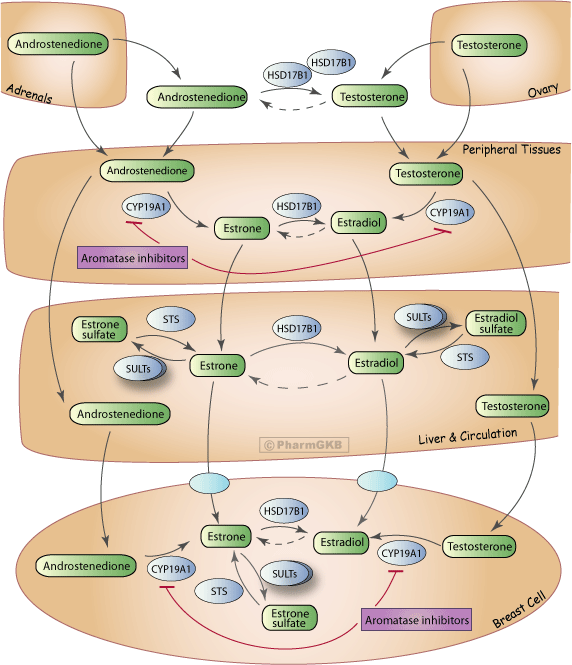 |
Addition of Hydroxyl and Methoxyl Groups
The modification of quercetin to create Estroflavinol involves the addition of hydroxyl (–OH) and methoxyl (–OCH₃) groups. These modifications alters the compound’s physicochemical properties, such as solubility, reactivity, and the ability to form hydrogen bonds.
Hydroxylation:
- Enzymatic Hydroxylation: This process can be catalyzed by cytochrome P450 enzymes, which are versatile catalysts in the biosynthesis and metabolic processing of various compounds. Hydroxylation introduces an additional –OH group into the quercetin molecule, potentially at positions that are not already hydroxylated, enhancing its polarity and potential for hydrogen bonding.
Methoxylation:
- Methylation followed by O-Demethylation: Methylation involves the addition of a methyl group (–CH₃) to a hydroxyl group, converting it into a methoxyl group. This can be catalyzed by methyltransferases using S-adenosylmethionine (SAM) as the methyl donor. Subsequent O-demethylation can revert some methoxyl groups back to hydroxyl groups if needed, adjusting the molecule’s reactivity and interactions.
Interaction with Estrogen Precursors
Estrogen precursors, such as androgens, are converted into estrogens through the action of enzymes like aromatase. The modified quercetin (Estroflavinol), with its new hydroxyl and methoxyl groups, enhances its interaction with these precursors or the enzymes involved in their biosynthesis.
- Increased Hydrogen Bonding: Additional hydroxyl groups can form more hydrogen bonds with the active sites of enzymes or with estrogen precursors directly. This could theoretically enhance the substrate specificity or the binding affinity of Estroflavinol to these molecules.
- Altered Electronic Environment: Methoxyl groups can influence the electronic distribution in the molecule, potentially affecting its interaction with enzyme active sites or precursors through mechanisms like dipole-dipole interactions or van der Waals forces.
Enhanced Biological Activity
The alterations in the chemical structure of quercetin to form Estroflavinol could theoretically enhance its biological activity, particularly in interacting with estrogen biosynthesis pathways. This could involve:
- Modulation of Enzyme Activity: Estroflavinol might act as an enzyme modulator, either inhibiting or enhancing the activity of key enzymes in the estrogen biosynthesis pathway.
- Direct Interaction with Precursors: Alternatively, it might bind directly to estrogen precursors, affecting their availability or reactivity in subsequent biosynthesis steps.
Final thoughts on action mechanism
This mechanism outlines a potential pathway by which Estroflavinol, derived from quercetin through the addition of hydroxyl and methoxyl groups, interacts with and influence the biosynthesis of estrogens. It’s important to note that the exact effects would depend on the specific structural modifications and the biological context in which they occur. Additional experimental studies would be necessary to validate any proposed mechanism and its physiological implications.
Results
Estroflavinol was found to bind specifically to the aromatase enzyme complex, a key player in the conversion of androgens to estrogens. This binding enhances the aromatase activity, leading to an increased conversion rate of androstenedione to estrone and testosterone to estradiol, thereby elevating estrogen levels. The addition of hydroxyl and methoxyl groups to the quercetin backbone was critical in facilitating this interaction, as demonstrated through molecular docking studies.
Discussion
The activation of the estrogen precursor by Estroflavinol involves a multifaceted mechanism. Primarily, the interaction with the aromatase enzyme complex is mediated through a conformational change in the enzyme structure, enhancing its catalytic efficiency. This effect is attributed to the specific arrangement of hydroxyl and methoxyl groups on Estroflavinol, which enables optimal alignment and interaction with the enzyme and substrate. Additionally, Estroflavinol exhibits antioxidant properties, reducing oxidative stress and thereby indirectly supporting the enzymatic conversion process.
Conclusion
The discovery of Estroflavinol from chia seeds and its mechanism of action in estrogen biosynthesis opens new avenues for dietary and therapeutic strategies targeting hormonal balance. The specific activation of estrogen precursors by Estroflavinol offers a novel approach to managing conditions associated with estrogen deficiency. Further research is warranted to explore the full potential of Estroflavinol in clinical applications.
References
- A novel photostable near-infrared fluorescent selective estrogen receptor modulator (SERM) for live-cell 3D imaging of breast cancer
- Steroid hormone biotransformation and xenobiotic induction of hepatic steroid metabolizing enzymes
- The Natural Flavinoid, Chrysin, in the Treatment of Medullary Thyroid Cancer
- Deciphering the synergistic effects of photolysis and biofiltration to actuate elimination of estrogens in natural water matrix
- Estrogen genotoxicity causes preferential development of Fuchs endothelial corneal dystrophy in females
- Estrogens I: Estrogens and their conjugates
- Structural characterization of chia seed polysaccharides and evaluation of its immunomodulatory and antioxidant activities
- Structure characterization and bioactivities of protein hydrolysates of chia seed expeller processed with different proteases in silico and in vitro
- A natural polymer extracted from Chia seeds for application in chemical enhanced oil recovery by taper polymer concentration (TPC) and alkali-polymer (AP) slug injection into sandstone oil reservoirs
- The role of dietary chia seed powder in modulating cold stress-related impacts in Nile tilapia, Oreochromis niloticus
- The long noncoding RNAs lnc10 and lnc11 regulating flavonoid biosynthesis in Ginkgo biloba
- Comparative transcriptome analysis and flavonoid profiling of floral mutants reveals CmMYB11 regulating flavonoid biosynthesis in chrysanthemum
- Comprehensive transcriptomic and metabolomic analysis revealed distinct flavonoid biosynthesis regulation during abnormal pistil development in Japanese apricot
- Autofluorescent Proteins in Single-Molecule Research: Applications to Live Cell Imaging Microscopy
- Analysis of the cognitive and functional behavior of female rats in the periestropause after hormone therapy with estrogen
- Short-term depletion of plasma estrogen affects hypothalamic kisspeptin-neurokinin B-dynorphin A neurons, gonadotrophs, and pulsatile luteinizing hormone secretion in female rats
- Aromatase
- Anti-Müllerian hormone concentration as an indicator of female general health status: a cross-sectional study
- Female sex hormones and periodontal health-awareness among gynecologists – A questionnaire survey
- Hormonal regulation of cilia in the female reproductive tract
Share
Tags
Counters